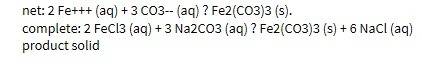Chemistry, 29.06.2019 14:30 brodysalander007
32. write the net ionic equation for the reaction of sodium phosphate with iron (iii) chloride. include the complete ionic equation and label the spectator ions. also be sure to label the reactants as products as solid, liquid, gas, or aqueous solutions. (5 points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
32. write the net ionic equation for the reaction of sodium phosphate with iron (iii) chloride. incl...
Questions

Mathematics, 05.05.2020 09:25

English, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25

Biology, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25



Physics, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25




English, 05.05.2020 09:25



Biology, 05.05.2020 09:25

Mathematics, 05.05.2020 09:25