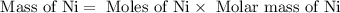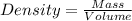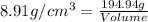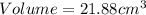Chemistry, 29.06.2019 14:30 school4life110
27. the density of nickel is 8.91 g/cm3. how large a cube, in cm3, would contain 2.00 x 10^24 atoms of nickel? use dimensional analysis to solve and show all work including units on every number! (5 points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
27. the density of nickel is 8.91 g/cm3. how large a cube, in cm3, would contain 2.00 x 10^24 atoms...
Questions


English, 24.05.2020 01:05



Mathematics, 24.05.2020 01:05




Mathematics, 24.05.2020 01:05

Mathematics, 24.05.2020 01:05

Medicine, 24.05.2020 01:05




Mathematics, 24.05.2020 01:05




English, 24.05.2020 01:05

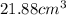
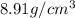
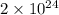
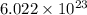 atoms form 1 mole of nickel
atoms form 1 mole of nickel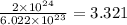 moles of nickel
moles of nickel