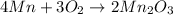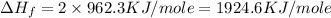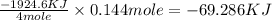Chemistry, 29.06.2019 20:30 ellycleland16
The standard enthalpy of formation of mn2o3 is â962.3 kj/mol. how much heat energy is liberated when 7.9 grams of manganese are oxidized by oxygen gas to mn2o3 at standard state conditions? answer in units of kj.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
The standard enthalpy of formation of mn2o3 is â962.3 kj/mol. how much heat energy is liberated when...
Questions



Advanced Placement (AP), 16.01.2021 02:40



Computers and Technology, 16.01.2021 02:40




Chemistry, 16.01.2021 02:50


Mathematics, 16.01.2021 02:50

Social Studies, 16.01.2021 02:50

Mathematics, 16.01.2021 02:50

Mathematics, 16.01.2021 02:50


Geography, 16.01.2021 02:50



Geography, 16.01.2021 02:50

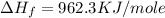 (for 1 mole)
(for 1 mole)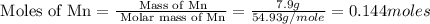
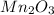 is represented as,
is represented as,