
Chemistry, 29.06.2019 20:30 nickocasamplonp6mlob
Sucrose (c12h22o11, table sugar) is oxidized in the body by o2 via a complex set of reactions that ultimately produces co2(g) and h2o(l) and releases 5.16 ã 103 kj of heat per mole of sucrose. (a) write a balanced thermochemical equation for this reaction. include the physical state of each reactant and product. â enter your answer for î´hrxn in scientific notation. î´hrxn

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Sucrose (c12h22o11, table sugar) is oxidized in the body by o2 via a complex set of reactions that u...
Questions

History, 15.04.2020 23:57


Spanish, 15.04.2020 23:57








English, 15.04.2020 23:58


Physics, 15.04.2020 23:58

Mathematics, 15.04.2020 23:58

English, 15.04.2020 23:58



Mathematics, 15.04.2020 23:58


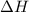 ) in a chemical reaction.
) in a chemical reaction.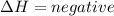 energy is released.
energy is released.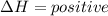 energy is absorbed.
energy is absorbed.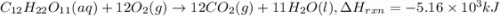
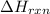 that is
that is  .
.

