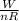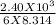Chemistry, 30.06.2019 00:00 scottbrandon653
Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. the initial temperature of the gas is 27.0c and the pressure is constant. as part of a machine design project, calculate the final temperature of the gas after it has done 2.40 * 103 j of work.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. the initial tem...
Questions

Mathematics, 07.12.2019 01:31


Mathematics, 07.12.2019 01:31

Mathematics, 07.12.2019 01:31

Social Studies, 07.12.2019 01:31

Mathematics, 07.12.2019 01:31

Social Studies, 07.12.2019 01:31

Mathematics, 07.12.2019 01:31

English, 07.12.2019 01:31


Computers and Technology, 07.12.2019 01:31




Mathematics, 07.12.2019 01:31

Mathematics, 07.12.2019 01:31


Mathematics, 07.12.2019 01:31


Mathematics, 07.12.2019 01:31