
Chemistry, 01.07.2019 08:30 edeliz2804
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced when 65.2 g of al is reacted with an excess (unlimited) supply of fe2o3?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced...
Questions

Computers and Technology, 01.09.2021 17:00





Mathematics, 01.09.2021 17:00

History, 01.09.2021 17:00

Mathematics, 01.09.2021 17:00

Mathematics, 01.09.2021 17:00


Biology, 01.09.2021 17:00


Biology, 01.09.2021 17:00







Biology, 01.09.2021 17:00

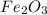 .
.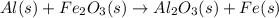

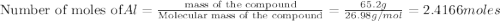
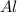 reacts with one mole of
reacts with one mole of 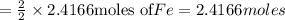
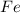 produced =
produced =


