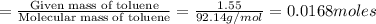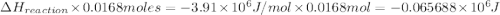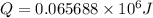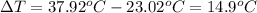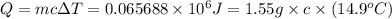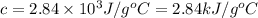Chemistry, 01.07.2019 11:30 samanthashade3434
The combustion of toluene has a δerxn of −3.91 × 103kj/mol. when 1.55 g of toluene (c7h8) undergoes combustion in a bomb calorimeter, the temperature rises from 23.02 ∘c to 37.92 ∘c. find the heat capacity of the bomb calorimeter.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1


Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
The combustion of toluene has a δerxn of −3.91 × 103kj/mol. when 1.55 g of toluene (c7h8) undergoes...
Questions





Mathematics, 08.10.2019 11:10

Mathematics, 08.10.2019 11:10




Biology, 08.10.2019 11:10


Social Studies, 08.10.2019 11:10



Social Studies, 08.10.2019 11:10




History, 08.10.2019 11:10

History, 08.10.2019 11:10

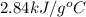 .
.