
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the molecules. carbon tetrachloride ccl4 is also nonploar but is made larger molecules that are more strongly affected by london dispersion forces. based on this information cs2 likely has a melting point and a vapor pressure when compared with ccl4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1
You know the right answer?
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the mole...
Questions


Mathematics, 17.11.2020 19:00

History, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

Arts, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

History, 17.11.2020 19:00


Mathematics, 17.11.2020 19:00


English, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

Advanced Placement (AP), 17.11.2020 19:00




Mathematics, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

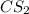 where as the vapor pressure of
where as the vapor pressure of ![CS_2[/tex[ is higher than [tex]CCl_4](/tpl/images/0043/9801/99b5e.png) .
.

