

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Adding naoh to an aqueous solution containing ni2+ results in the precipitation ofni(oh)2. the stand...
Questions

Mathematics, 03.12.2021 07:30



Mathematics, 03.12.2021 07:30

Mathematics, 03.12.2021 07:30


Business, 03.12.2021 07:30




Mathematics, 03.12.2021 07:30

Mathematics, 03.12.2021 07:30


Chemistry, 03.12.2021 07:30

Computers and Technology, 03.12.2021 07:30

English, 03.12.2021 07:30

English, 03.12.2021 07:30

History, 03.12.2021 07:30

Mathematics, 03.12.2021 07:30

Mathematics, 03.12.2021 07:30

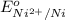 = +0.25 V
= +0.25 V![K_{sp}=[Ni^{2+}][OH^-]^2=1.5\times 10^{-16}](/tpl/images/0048/3070/43bf5.png)
![pH=-log[H^+]=14](/tpl/images/0048/3070/4f928.png)
![[H^+]=10^{-14}](/tpl/images/0048/3070/eec66.png)
![K_w=[H^+][OH^-]=10^{-14}](/tpl/images/0048/3070/f3553.png)
![[OH^-]=\frac{K_w}{[H^+]}=\frac{10^{-14}}{10^{-14}}=1](/tpl/images/0048/3070/8d077.png)
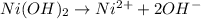
![K_{sp}=[Ni^{2+}][OH^-]^2](/tpl/images/0048/3070/c86a1.png)
![[Ni^{2+}]](/tpl/images/0048/3070/2f342.png) ion.
ion.^2](/tpl/images/0048/3070/f235c.png)
![[Ni^{2+}]=1.5\times 10^{-16}](/tpl/images/0048/3070/989b5.png)
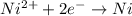
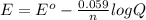
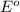 = standard electrode potential of the cell
= standard electrode potential of the cell![E=E^o_{Ni^{2+}/Ni}-\frac{0.059}{n}log\frac{1}{[Ni^{2+}]}](/tpl/images/0048/3070/64772.png)
![E=+0.25V-\frac{0.059}{2}log\frac{1}{[1.5\times 10^{-16}]}](/tpl/images/0048/3070/b7e57.png)


