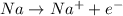Chemistry, 04.07.2019 00:30 haddockc2689
The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction reaction a)loses electrons and loses potential energy b)gains electrons and gains potential c)energy gains electrons and loses potential energy d)loses electrons and gains potential energy

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction...
Questions

Health, 05.02.2020 04:47




Mathematics, 05.02.2020 04:47






Biology, 05.02.2020 04:47

Mathematics, 05.02.2020 04:47



Mathematics, 05.02.2020 04:47


History, 05.02.2020 04:47