
Chemistry, 04.07.2019 22:00 jlayne0605
Which substance in this redox reaction is the oxidizing agent? cu + 2agno3 → 2ag + cu(no3)2 a. n b. agno3 c. cu d. no3− e. cu(no3)2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Which substance in this redox reaction is the oxidizing agent? cu + 2agno3 → 2ag + cu(no3)2 a. n b...
Questions

Business, 26.05.2020 02:58


Mathematics, 26.05.2020 02:58


History, 26.05.2020 02:58

Mathematics, 26.05.2020 02:58

Mathematics, 26.05.2020 02:58


Geography, 26.05.2020 02:58

Biology, 26.05.2020 02:58

Computers and Technology, 26.05.2020 02:58


Mathematics, 26.05.2020 02:58





English, 26.05.2020 02:59

Chemistry, 26.05.2020 02:59

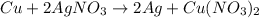
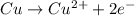
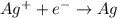
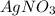 is considered as an oxidizing agent and therefore the correct answer is Option B.
is considered as an oxidizing agent and therefore the correct answer is Option B.

