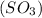Chemistry, 04.07.2019 22:00 masaiyahrhemanow
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g) a. no increases the rate at which so3 molecules are formed. b. no reacts with so3 to produce more so2 molecules. c. no decreases collisions between the so2 and o2 molecules. d. no increases the concentration of the so2 and o2 molecules. e. no increases the activation energy of the so2 and o2 molecules.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

You know the right answer?
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g...
Questions

Chemistry, 11.02.2021 14:00

History, 11.02.2021 14:00

English, 11.02.2021 14:00

History, 11.02.2021 14:00



Mathematics, 11.02.2021 14:00

Physics, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00



Mathematics, 11.02.2021 14:00

Chemistry, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00


Chemistry, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

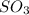 molecules are formed.
molecules are formed.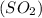 and
and 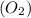 undergo collision and can cross the energy barrier and convert to products
undergo collision and can cross the energy barrier and convert to products