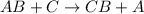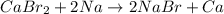Chemistry, 05.07.2019 22:30 codeyhatch142
Based on the reactivities of the elements involved, which reaction will form products that are more stable than the reactants? a. 2albr3 + 3zn → 3znbr2 + 2al b. cabr2 + 2na → 2nabr + ca c. mgbr2 + h2 → 2hbr + mg d. babr2 + ca → cabr2 + ba e. 2libr + ba → babr2 + 2libased on the reactivities of the elements involved, which reaction will form products that are more stable than the reactants? a. 2albr3 + 3zn → 3znbr2 + 2al b. cabr2 + 2na → 2nabr + ca c. mgbr2 + h2 → 2hbr + mg d. babr2 + ca → cabr2 + ba e. 2libr + ba → babr2 + 2li

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Based on the reactivities of the elements involved, which reaction will form products that are more...
Questions



Health, 23.07.2019 03:00


Social Studies, 23.07.2019 03:00


Mathematics, 23.07.2019 03:00

Mathematics, 23.07.2019 03:00


Physics, 23.07.2019 03:00

Mathematics, 23.07.2019 03:00






Mathematics, 23.07.2019 03:00



History, 23.07.2019 03:00