
Several solutions of nickel (ii) sulfate ranging from 1.00m to 0.200 m are exposed to light in a colorimeter and their absorbance is recorded in a beer-lambert law experiment. why does a solution of nickel(ii) sulfate represent a suitable solution to be studied in this experiment, whereas similar solutions of sodium chloride do not?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Several solutions of nickel (ii) sulfate ranging from 1.00m to 0.200 m are exposed to light in a col...
Questions

Biology, 31.01.2020 10:57

Mathematics, 31.01.2020 10:57





History, 31.01.2020 10:57




Computers and Technology, 31.01.2020 10:57

History, 31.01.2020 10:57



English, 31.01.2020 10:57



Mathematics, 31.01.2020 10:57


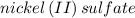 is green in color but a solution of sodium chloride is colorless that is why
is green in color but a solution of sodium chloride is colorless that is why 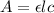
 = molar absorptivity
= molar absorptivity

