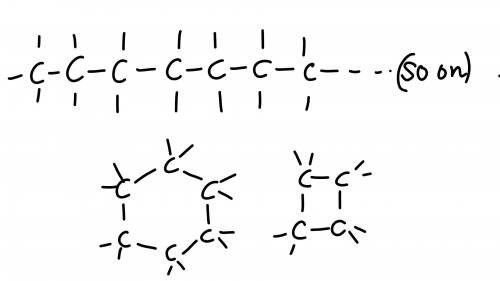Chemistry, 06.07.2019 05:30 sliverx201
25 pointsquestion how does the structure of a carbon atom enable it to form large molecules? available choices [a] each carbon atom can be stable with one, two, three, or four bonds because of how its valence electrons are arranged. [b] each carbon atom can bond with several other carbon atoms because of how many valence electrons it has. [c] each carbon atom donates its electrons to other atoms, including atoms of noble gases and halogens. [d] each carbon atom forms either double or triple bonds with surrounding hydrogen atoms. try your best, this is important, 25 points and brainliest answer if you get it right!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Assume that the variables x and y are inversely related. if k = 18, what is the value of y for each of the following points? be sure and record your data to be used in the following problem.
Answers: 2



Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
25 pointsquestion how does the structure of a carbon atom enable it to form large molecules? availab...
Questions

Mathematics, 04.12.2021 01:10

Mathematics, 04.12.2021 01:10


History, 04.12.2021 01:10




Business, 04.12.2021 01:10


Mathematics, 04.12.2021 01:10

Mathematics, 04.12.2021 01:10

Mathematics, 04.12.2021 01:10

Biology, 04.12.2021 01:10

Mathematics, 04.12.2021 01:10





Mathematics, 04.12.2021 01:10

Mathematics, 04.12.2021 01:10