

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2


Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Atank contains a mixture of 52.5g oxygen gas and 65.1g carbon dioxide gas at 27c. the total pressure...
Questions

Social Studies, 20.07.2019 03:00


Mathematics, 20.07.2019 03:00

History, 20.07.2019 03:00

English, 20.07.2019 03:00


Mathematics, 20.07.2019 03:00

Mathematics, 20.07.2019 03:00




Mathematics, 20.07.2019 03:00


History, 20.07.2019 03:00

Mathematics, 20.07.2019 03:00

Spanish, 20.07.2019 03:00


Mathematics, 20.07.2019 03:00

History, 20.07.2019 03:00

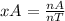
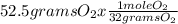 = 1.641 mol O₂
Moles of CO₂ =
= 1.641 mol O₂
Moles of CO₂ =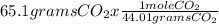 = 1.479 mol CO₂
= 1.479 mol CO₂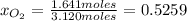
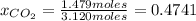
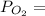 0.5259× 9.21 atm= 4.84 atm
0.5259× 9.21 atm= 4.84 atm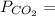 0.4741× 9.21 atm= 4.37 atm
0.4741× 9.21 atm= 4.37 atm


