Chemistry, 09.07.2019 12:30 doggosbepis
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of sulfur trioxide (in g) produced when 100.0 g of each reactant is present.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of su...
Questions



Mathematics, 21.04.2020 00:57



Physics, 21.04.2020 00:57






Biology, 21.04.2020 00:58


Chemistry, 21.04.2020 00:58






Mathematics, 21.04.2020 00:58

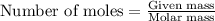 .....(1)
.....(1)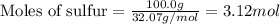
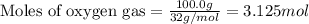
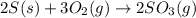
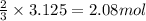 of sulfur metal
of sulfur metal