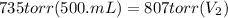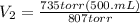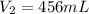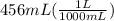Chemistry, 09.07.2019 20:30 saladdressing1425
Acontainer holds 500. ml of co2 at 20.° c and 735 torr. what will be the volume of the co2 if the pressure is increased to 807 torr? (0.456 l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

You know the right answer?
Acontainer holds 500. ml of co2 at 20.° c and 735 torr. what will be the volume of the c...
Questions

History, 17.03.2022 14:40




Mathematics, 17.03.2022 14:50



Mathematics, 17.03.2022 14:50

Mathematics, 17.03.2022 14:50




Social Studies, 17.03.2022 14:50


World Languages, 17.03.2022 14:50

Mathematics, 17.03.2022 14:50

Mathematics, 17.03.2022 14:50


English, 17.03.2022 15:00

Social Studies, 17.03.2022 15:00

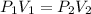
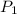 = 735 torr
= 735 torr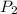 = 807 torr
= 807 torr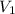 = 500. mL
= 500. mL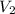 = ?
= ?