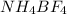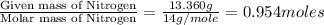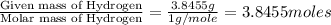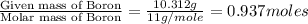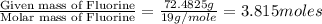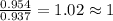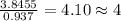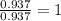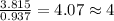Chemistry, 09.07.2019 22:00 einstein101
Anew compound contains nitrogen, hydrogen, boron, and fluorine. the assay values are: nitrogen, 13.360%; hydrogen, 3.8455%; boron, 10.312%. determine its empirical formula.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Anew compound contains nitrogen, hydrogen, boron, and fluorine. the assay values are: nitrogen, 13....
Questions



History, 12.01.2021 19:20


History, 12.01.2021 19:20




English, 12.01.2021 19:20

Mathematics, 12.01.2021 19:20

English, 12.01.2021 19:20

Advanced Placement (AP), 12.01.2021 19:20


Mathematics, 12.01.2021 19:20

Health, 12.01.2021 19:20

Mathematics, 12.01.2021 19:20

Mathematics, 12.01.2021 19:20

Mathematics, 12.01.2021 19:20