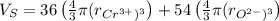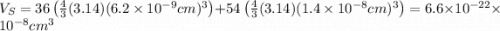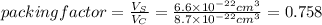Chemistry, 09.07.2019 22:00 nancye2008
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 nm. if the density of this material is 5.22 g/cm3, calculate its atomic packing factor. the atomic weights of cr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 n...
Questions


Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Biology, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01




Mathematics, 12.10.2020 21:01


English, 12.10.2020 21:01

Chemistry, 12.10.2020 21:01

Biology, 12.10.2020 21:01

Arts, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

History, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

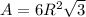
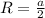
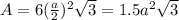
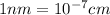
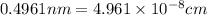
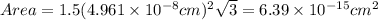
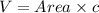
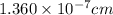
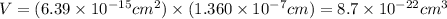
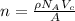
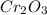 is 151.99 g/mol.
is 151.99 g/mol.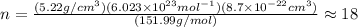
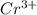 and
and 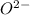 is 62 pm and 140 pm respectively.
is 62 pm and 140 pm respectively. 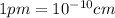
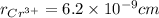
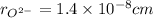
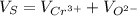
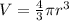 ,
,