Chemistry, 10.07.2019 01:30 isaiahmichel93081
3: you place 2.56 g of caco3(fw = 100.09 g/mol) in a beaker containing 250. ml of 0.125 m hcl. what the reactions has ceased, what mass of cacl2(fw = 110.98 g/mol) can be produced? the unbalancedequation i

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
3: you place 2.56 g of caco3(fw = 100.09 g/mol) in a beaker containing 250. ml of 0.125 m hcl. what...
Questions






Biology, 13.09.2019 22:30


Biology, 13.09.2019 22:30

Biology, 13.09.2019 22:30










Chemistry, 13.09.2019 22:30

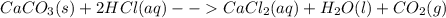
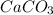 =
=
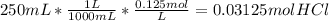
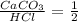
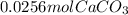 per 0.03125 mol HCl
per 0.03125 mol HCl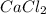 =0.03125mol HCl *
=0.03125mol HCl *