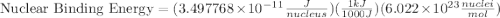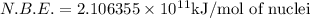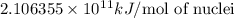Chemistry, 10.07.2019 12:30 janicemaxwell123
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and neutrons if an aluminum-27 atom has a mass of 26.9815386 amu? (the mass of an electron is 5.485799×10−4 amu, the mass of a proton is 1.0072765 amu, and the mass of a neutron is 1.0086649 amu.) express your answer using six significant figures. g?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and ne...
Questions

Mathematics, 20.07.2020 20:01

Mathematics, 20.07.2020 20:01


Mathematics, 20.07.2020 20:01

Mathematics, 20.07.2020 20:01





Computers and Technology, 20.07.2020 20:01

Mathematics, 20.07.2020 20:01

English, 20.07.2020 20:01


Mathematics, 20.07.2020 20:01

History, 20.07.2020 20:01

Computers and Technology, 20.07.2020 20:01


Mathematics, 20.07.2020 20:01


Mathematics, 20.07.2020 20:01

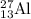 nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from
nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from 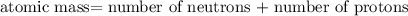 .
. 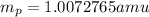 and
and 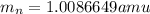 , we can calculate the mass of the isotope.
, we can calculate the mass of the isotope.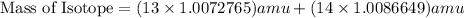
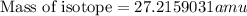
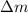 = mass of isotope - atomic mass.
= mass of isotope - atomic mass.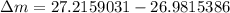
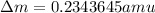
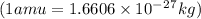
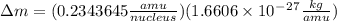
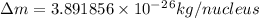
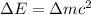
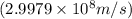
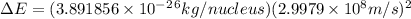
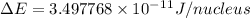
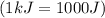 and converting individual particles into moles, we need to multiply it by avagadro's number that is
and converting individual particles into moles, we need to multiply it by avagadro's number that is 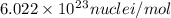 .
.