Chemistry, 11.07.2019 07:30 Jaylen52709
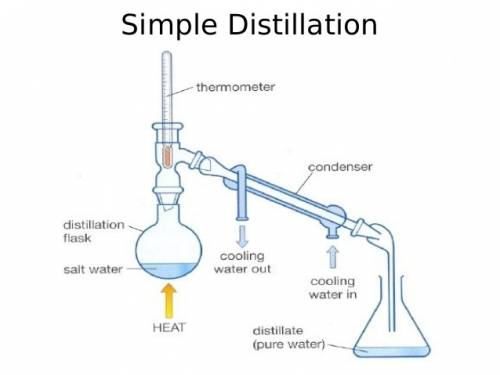
Astudent carried out a simple distillation on a compound known to boil at 124 oc and they reported a boiling point of 116-117 o analysis of the compound showed it was pure and calibration of the thermometer indicated that it was accurate. what procedural error might the student have made in setting up the distillation?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Astudent carried out a simple distillation on a compound known to boil at 124 oc and they reported a...
Questions



Biology, 25.07.2019 04:30



Mathematics, 25.07.2019 04:30

English, 25.07.2019 04:30


Mathematics, 25.07.2019 04:30


Mathematics, 25.07.2019 04:30


Social Studies, 25.07.2019 04:30



Biology, 25.07.2019 04:30

Advanced Placement (AP), 25.07.2019 04:30

Geography, 25.07.2019 04:30

Mathematics, 25.07.2019 04:30