
Chemistry, 11.07.2019 19:00 rainingsouls
You are presented with a white solid and told that due to careless labeling it is not clear if the substance is barium chloride, lead chloride, or zinc chloride. when you transfer the solid to a beaker and add water, the solid dissolves to give a clear solution. next a na2so4(aq) solution is added and a white precipitate forms. what is the identity of the unknown white solid?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
You are presented with a white solid and told that due to careless labeling it is not clear if the s...
Questions

Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00



Mathematics, 04.10.2021 14:00



Chemistry, 04.10.2021 14:00





Mathematics, 04.10.2021 14:00





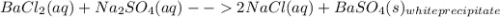
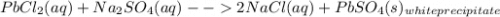
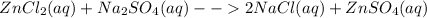
 + Na
+ Na
 2 NaCl + BaSO₄
2 NaCl + BaSO₄ 


