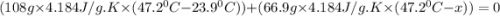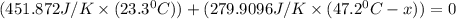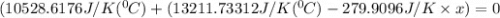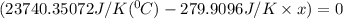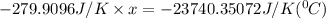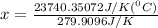Chemistry, 12.07.2019 09:00 Elephants12
When 108 g of water at a temperature of 23.9 °c is mixed with 66.9 g of water at an unknown temperature, the final temperature of the resulting mixture is 47.2 °c. what was the initial temperature of the second sample of water? (the specific heat capacity of liquid water is 4.184 j/g ⋅

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2


Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
When 108 g of water at a temperature of 23.9 °c is mixed with 66.9 g of water at an unknown temperat...
Questions

Mathematics, 16.06.2020 18:57

English, 16.06.2020 18:57


English, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

English, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57


Mathematics, 16.06.2020 18:57

English, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

Chemistry, 16.06.2020 18:57



Physics, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

Mathematics, 16.06.2020 18:57

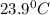
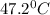
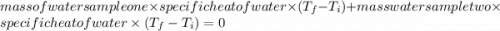
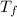 = final temperature
= final temperature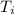 = initial temperature
= initial temperature