
Chemistry, 12.07.2019 09:00 gracieorman4
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapor. calculate the moles of water produced by the reaction of 0.060mol of oxygen. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 10:30
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2
You know the right answer?
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapo...
Questions




Mathematics, 02.03.2021 18:20

Chemistry, 02.03.2021 18:20

Chemistry, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

History, 02.03.2021 18:20

Business, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20





Mathematics, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

Social Studies, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

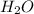 ) produced by the reaction of 0.060mol of oxygen.
) produced by the reaction of 0.060mol of oxygen.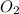 ) given = 0.060 mole
) given = 0.060 mole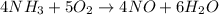
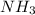 to give 4 moles of NO and 6 moles of
to give 4 moles of NO and 6 moles of 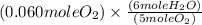
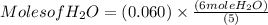
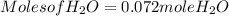
 4 NO + 6 H₂O
4 NO + 6 H₂O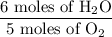 Moles of H₂O = 0.060 moles of O₂ x
Moles of H₂O = 0.060 moles of O₂ x 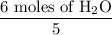 Moles of H₂O = 0.072 moles of H₂O.
Moles of H₂O = 0.072 moles of H₂O. 

