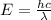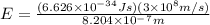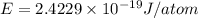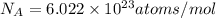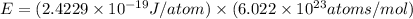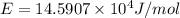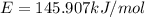Chemistry, 12.07.2019 19:30 yarrito20011307
Assume a hydrogen atom has an electron in the n-level corresponding to the month of your birthday (jan = 1, feb = 2, etc.) calculate the ionization energy of that hydrogen atom. hint: the ionization energy is defined as the energy needed to completely remove an electron from an atom, ion, or molecule.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1


Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
You know the right answer?
Assume a hydrogen atom has an electron in the n-level corresponding to the month of your birthday (j...
Questions








Mathematics, 06.05.2020 07:17






Mathematics, 06.05.2020 07:17






Biology, 06.05.2020 07:18

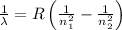
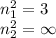
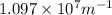
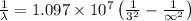
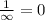
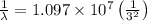
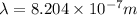
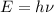 and
and 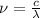
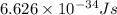 (Planck's constant)
(Planck's constant)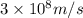 (Speed of light)
(Speed of light) in Energy formula, we get
in Energy formula, we get