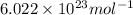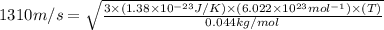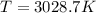Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal to 44.0 g/mol ) in a flame is found to be 1310 m/s. What temperature T does this represent? The Boltzmann constant is k=1.38×10−23 J/K and Avogadro's number is A=6.022×1023 mol−1.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal...
Questions

Mathematics, 20.09.2019 19:10

History, 20.09.2019 19:10








Computers and Technology, 20.09.2019 19:10




Mathematics, 20.09.2019 19:10






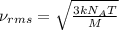
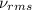 = root mean square speed = 1310 m/s
= root mean square speed = 1310 m/s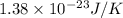
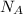 = Avogadro’s number =
= Avogadro’s number =