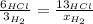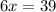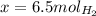Chemistry, 14.07.2019 22:30 maryalice2002
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can be formed? 2 al + 6hcl → 2 alcl3 + 3 h2 select one: a. al is the limiting reactant, 9.0 mol h2 can be formed b. hcl is the limiting reactant, 6.5 mol h2 can be formed c. al is the limiting reactant, 6.0 mol h2 can be formed d. hcl is the limiting reactant, 4.3 mol h2 can be formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can b...
Questions

Biology, 12.11.2020 06:00


Mathematics, 12.11.2020 06:00


French, 12.11.2020 06:00


Social Studies, 12.11.2020 06:00



Mathematics, 12.11.2020 06:00



Computers and Technology, 12.11.2020 06:00




Mathematics, 12.11.2020 06:00



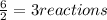
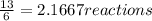
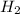 that are produced with 13 moles of HCl.
that are produced with 13 moles of HCl.