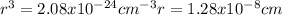Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Copper has a face-centered cubic unit cell. the density of copper is 8.96 g/cm3. calculate a value f...
Questions








Chemistry, 11.04.2020 03:45



Mathematics, 11.04.2020 03:45

Mathematics, 11.04.2020 03:45



Mathematics, 11.04.2020 03:46





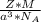
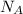 is Avogadro's number, and a is the edge length.
is Avogadro's number, and a is the edge length.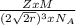
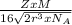
 =
=