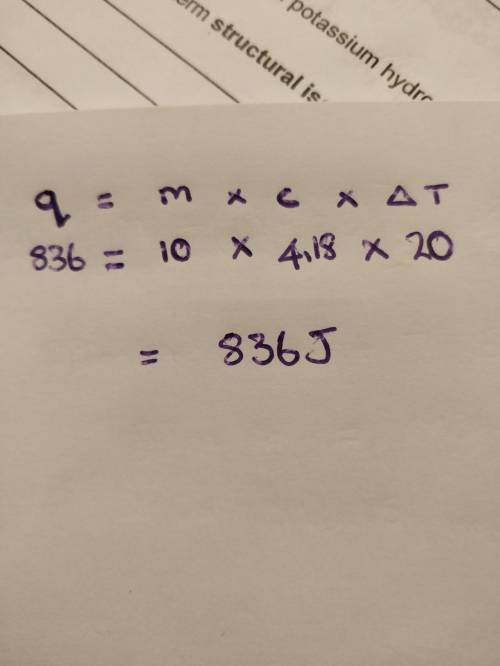Chemistry, 19.07.2019 06:00 johnkings140
What amount of heat is required to raise the temperature of 10.0 g of water from 15.0°c to 35.0°c? the specific heat of water is 4.18 j/g•°c. answer with 3 significant figures.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
What amount of heat is required to raise the temperature of 10.0 g of water from 15.0°c to 35.0°c?...
Questions


Chemistry, 21.09.2019 09:00


Mathematics, 21.09.2019 09:00

Chemistry, 21.09.2019 09:00

Mathematics, 21.09.2019 09:00

Mathematics, 21.09.2019 09:00




Mathematics, 21.09.2019 09:00


Biology, 21.09.2019 09:00

Mathematics, 21.09.2019 09:00

Chemistry, 21.09.2019 09:00

Spanish, 21.09.2019 09:00


Mathematics, 21.09.2019 09:00

Social Studies, 21.09.2019 09:00