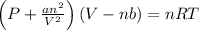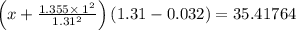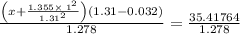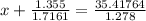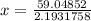Social Studies, 01.10.2019 00:30 92835304629
Calculate the pressure exerted by ar for a molar volume of 1.31 l mol–1 at 426 k using the van der waals equation of state. the van der waals parameters a and b for ar are 1.355 bar dm6 mol–2 and 0.0320 dm3 mol–1, respectively. is the attractive or repulsive portion of the potential dominant under these conditions?

Answers: 2


Another question on Social Studies

Social Studies, 22.06.2019 09:00
Which shows the best path to find the number of centimeters in 1 yard?
Answers: 1

Social Studies, 22.06.2019 17:30
What was the result of president taft's "dollar diplomacy
Answers: 2

Social Studies, 23.06.2019 03:10
In a study, people were asked to imagine simple occasions that made them happy and to remember events that made them feel proud about themselves. it was observed that the subjects developed an increasingly positive attitude. these changes are known to be healthy, leading to greater physical activity and reducing unhealthy habits. based on this excerpt, we can conclude that a positive attitude .
Answers: 2

Social Studies, 23.06.2019 09:00
What federal law makes marijuana illegal i know it's a schedule 1 drug but what law specifically gives the federal government the ability to arrest or fine you over marijuana i have only been able to find info on the scheduling of the drug but it never says this or that is illegal it just says that it's a schedule 1 drug i need the wording exactly my english teacher says that if i don't have it my paper is null and void
Answers: 1
You know the right answer?
Calculate the pressure exerted by ar for a molar volume of 1.31 l mol–1 at 426 k using the van der w...
Questions


History, 10.10.2019 10:10




Social Studies, 10.10.2019 10:10



History, 10.10.2019 10:10

Mathematics, 10.10.2019 10:10

English, 10.10.2019 10:10

Mathematics, 10.10.2019 10:10



Mathematics, 10.10.2019 10:10




History, 10.10.2019 10:20