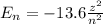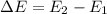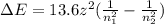How does quantum theory explain the emission spectra of atoms? a. electrons absorb the energy they need to jump to a higher energy level from any light source. b. a single, specific amount of energy is associated with each movement of an electron between energy levels. c. electrons gain and release energy in a slow, continuous fashion. d. the movement of an electron between energy levels occurs gradually as small amounts of energy are absorbed.

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:00
An open cart is moving along a straight frictionless horizontal track. when rain starts falling vertically into the cart, what happens to the speed of the cart?
Answers: 3

Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3


Physics, 23.06.2019 01:00
Nthe 19th century, women were expected to conform to a certain role that entailed a. gaining domestic skills and establishing a career b. going to college and getting married c. getting married and having children d. graduating from college and being a housewife
Answers: 1
You know the right answer?
How does quantum theory explain the emission spectra of atoms? a. electrons absorb the energy they...
Questions


English, 25.11.2019 10:31


Mathematics, 25.11.2019 10:31

Mathematics, 25.11.2019 10:31

World Languages, 25.11.2019 10:31


Social Studies, 25.11.2019 10:31



Mathematics, 25.11.2019 10:31


History, 25.11.2019 10:31

Mathematics, 25.11.2019 10:31

Social Studies, 25.11.2019 10:31

English, 25.11.2019 10:31

Business, 25.11.2019 10:31

Biology, 25.11.2019 10:31