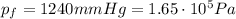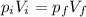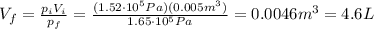Physics, 25.07.2019 14:00 oKINGDEROo
The pressure of 5.0 l of gas increases from 1.50 atm to 1240 mmhg. what is the final volume of the gas, assuming constant temperature?

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:30
How many molecules are present in 3 mols of silicon dioxide (sio2)?
Answers: 1

Physics, 22.06.2019 11:00
The government has declared that a hurricane is likely to hit the east coast. which government division is part of acf and provides guidance to citizens? a. job opportunities for low-income individuals b. office of human services emergency preparedness and response c. assets for independence d. low-income home energy assistance program
Answers: 1

Physics, 22.06.2019 13:00
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2

Physics, 22.06.2019 16:30
In a classical model of the hydrogen atom, the electron moves around the proton in a circular orbit of radius 0.053 nm. what is the electron's orbital frequency? what is the effective current of the electron?
Answers: 3
You know the right answer?
The pressure of 5.0 l of gas increases from 1.50 atm to 1240 mmhg. what is the final volume of the g...
Questions



Mathematics, 21.09.2020 14:01




Chemistry, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01


Chemistry, 21.09.2020 14:01

Arts, 21.09.2020 14:01



Mathematics, 21.09.2020 14:01


Mathematics, 21.09.2020 14:01


Geography, 21.09.2020 14:01

Health, 21.09.2020 14:01

Biology, 21.09.2020 14:01

 :
: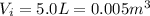
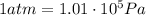 :
: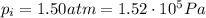
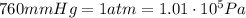 )
)