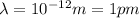Physics, 27.07.2019 14:00 shashi2728
How would you classify an em wave with a wavelength of 10 -12 m?

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:30
Matt is driving down 7th street. he drives 150 meters in 18 sec. assuming he doesn’t slow down or speed up, what is his speed?
Answers: 1

Physics, 22.06.2019 02:30
The particle in a two-dimensional well is a useful model for the motion of electrons around the indole ring (3), the conjugated cycle found in the side chain of tryptophan. we may regard indole as a rectangle with sides of length 280 pm and 450 pm, with 10 electrons in the conjugated p system. as in case study 9.1, we assume that in the ground state of the molecule each quantized level is occupied by two electrons. (a) calculate the energy of an electron in the highest occupied level. (b) calculate the frequency of radiation that can induce a transition between the highest occupied and lowest unoccupied levels. 9.27 electrons around the porphine ring (4), the conjugated macrocycle that forms the structural basis of the heme group and the chlorophylls. we may treat the group as a circular ring of radius 440 pm, with 20 electrons in the conjugated system moving along the perimeter of the ring. as in exercise 9.26, assume that in the ground state of the molecule quantized each level is occupied by two electrons. (a) calculate the energy and angular momentum of an electron in the highest occupied level. (b) calculate the frequency of radiation that can induce a transition between the highest occupied and lowest unoccupied levels.
Answers: 1

Physics, 22.06.2019 14:30
Exercise 2. find the wavelength of a photon emitted when an electron jumps from the n = 3 energy level down to the n = 2 energy level. where is this photon in the electromagnetic spectrum?
Answers: 3

Physics, 22.06.2019 17:30
Frequency of electromagnetic waves that a radio station is assigned is called
Answers: 1
You know the right answer?
How would you classify an em wave with a wavelength of 10 -12 m?...
Questions


Business, 16.07.2019 17:00


Business, 16.07.2019 17:00

Biology, 16.07.2019 17:00



Computers and Technology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00

History, 16.07.2019 17:00

History, 16.07.2019 17:00

Biology, 16.07.2019 17:00

Computers and Technology, 16.07.2019 17:00


Biology, 16.07.2019 17:00