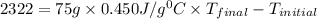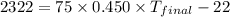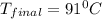Physics, 03.08.2019 16:00 AlaskaAirlines
Suppose that 0.95 g of water condenses on a 75.0 g block of iron that is initially at 22 â°c. if the heat released during condensation is used only to warm the iron block, what is the final temperature (in â°c) of the iron block? (assume a constant enthalpy of vaporization for water of 44.0 kj/mol.)

Answers: 2


Another question on Physics

Physics, 22.06.2019 01:30
John throws .4 kg ball with velocity of 18 m/s. hits .2 kg bottle and bottle flies 25 m/s. how fast is ball traveling after hitting the bottle?
Answers: 1

Physics, 22.06.2019 07:20
If 2 moles of co_2 at 2l and 500k are expanded reversibly to 20l, more work can be obtained from an adiabatic process than from an isothermal process. is the above statement true or false?
Answers: 3

Physics, 22.06.2019 08:30
The coefficient of friction is a number that represents the resistance to sliding between two in contact with one another.
Answers: 2

Physics, 22.06.2019 14:40
The experiment done in lab is repeated, using a ball that has unknown mass m. you plot your data in the form of f 2 versus m/l, with f in rev/s, m in kg, and l in m. your data falls close to a straight line that has slope 3.19 m/(kg · s2). use g = 9.80 m/s2 and calculate the mass m of the ball.
Answers: 1
You know the right answer?
Suppose that 0.95 g of water condenses on a 75.0 g block of iron that is initially at 22 â°c. if the...
Questions


Mathematics, 23.12.2019 05:31

English, 23.12.2019 05:31

History, 23.12.2019 05:31

Mathematics, 23.12.2019 05:31


History, 23.12.2019 05:31

Mathematics, 23.12.2019 05:31


Computers and Technology, 23.12.2019 05:31

Mathematics, 23.12.2019 05:31


Mathematics, 23.12.2019 05:31

History, 23.12.2019 05:31

Mathematics, 23.12.2019 05:31



Mathematics, 23.12.2019 05:31


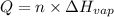
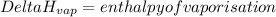
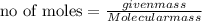
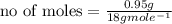
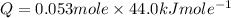
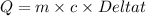
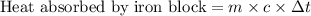
 =change in temperature
=change in temperature