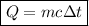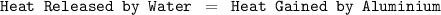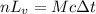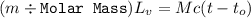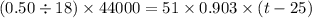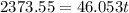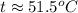Suppose that 0.50 g of water at 25 ∘c condenses on the surface of a 51-g block of aluminum that is initially at 25 ∘c. if the heat released during condensation goes only toward heating the metal, what is the final temperature (in ∘c) of the metal block? (the specific heat capacity of aluminum is 0.903 j/g∘c and the heat of vaporization of water at 25 ∘c is 44.0 kj/mol.)

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:00
Within a pendulum, as potential energy decreases, energy increases. a. heat b. kinetic c. frictional d. gravitational
Answers: 1

Physics, 22.06.2019 07:10
Polly is pushing a box across the floor with a force of 30 n. the force of gravity is –8 n, and the normal force is 8 n. which value could describe the force of friction if polly could not move the box?
Answers: 2

Physics, 22.06.2019 08:30
A40.0 l tank of ammonia has a pressure of 12.7 kpa. calculate the. volume of the ammonia if it’s pressure is changed to 8.4 kpa while its temperature remains constant.
Answers: 3

Physics, 22.06.2019 09:30
True or false graphs must include scales that increase by the same amount
Answers: 3
You know the right answer?
Suppose that 0.50 g of water at 25 ∘c condenses on the surface of a 51-g block of aluminum that is i...
Questions



Computers and Technology, 12.03.2020 23:59






Mathematics, 12.03.2020 23:59

Mathematics, 12.03.2020 23:59

Mathematics, 12.03.2020 23:59




Computers and Technology, 12.03.2020 23:59


History, 12.03.2020 23:59