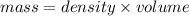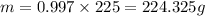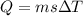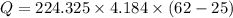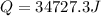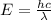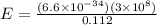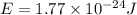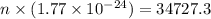Physics, 16.07.2019 15:10 dontcareanyonemo
Microwave ovens use microwave radiation to heat food. the microwaves are absorbed by the water molecules in the food, which is transferred to other components of the food. as the water becomes hotter, so does the food. part a suppose that the microwave radiation has a wavelength of 11.2 cm . how many photons are required to heat 225 ml of coffee from 25.0 ∘c to 62.0 ∘c? assume that the coffee has the same density, 0.997 g/ml , and specific heat capacity, 4.184 j/(g⋅k) , as water over this temperature range.

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:00
How much work would be needed to raise the payload from the surface of the moon (i.e., x = r) to an altitude of 5r miles above the surface of the moon (i.e., x = 6r)?
Answers: 2


Physics, 22.06.2019 22:50
Which of the following statements correctly describes a transformer?
Answers: 3

Physics, 23.06.2019 08:30
Which equation is mostly likely used to determine the acceleration from a velocity vs time graph
Answers: 1
You know the right answer?
Microwave ovens use microwave radiation to heat food. the microwaves are absorbed by the water molec...
Questions



History, 03.11.2020 22:20

Mathematics, 03.11.2020 22:20


English, 03.11.2020 22:20


English, 03.11.2020 22:20

Biology, 03.11.2020 22:20

Mathematics, 03.11.2020 22:20

Biology, 03.11.2020 22:20

Mathematics, 03.11.2020 22:20




Computers and Technology, 03.11.2020 22:20

Health, 03.11.2020 22:20

Arts, 03.11.2020 22:20

Advanced Placement (AP), 03.11.2020 22:20

Geography, 03.11.2020 22:20

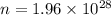 photons
photons