
Physics, 03.12.2021 22:40 almaga1979orfvwo
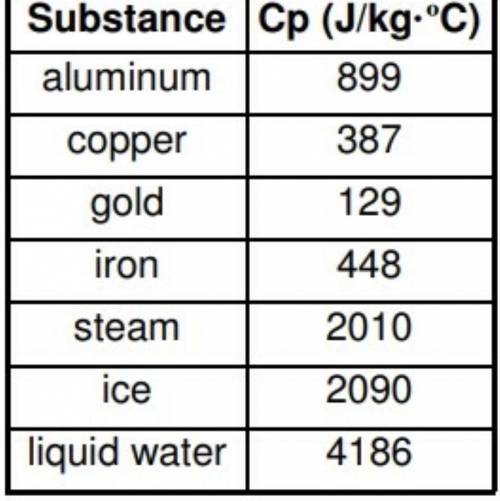
A 2.5 kg sample of water with an intial temperature of 98o C loses 375,000 J of heat. What is the final temperature of the water?
a
81.43o C
b
36.55o C
c
57.92o C
d
62.17o C

Answers: 2


Another question on Physics

Physics, 21.06.2019 21:30
Although light from the sun appears white, it is actually made up of a lot of different colors. this portion of the electromagnetic spectrum, we call white light is referred to as within the electromagnetic spectrum.
Answers: 1

Physics, 22.06.2019 10:00
Which is the correct symbol for an isotope of iodine with 53 protons and 78 neutrons?
Answers: 2

Physics, 22.06.2019 12:50
Assume you measured the mass of the cart to be (500 ± 1) g and the mass of the additional mass you put on the cart to be (500 ± 1) g as well. since the scale you are using in the lab cannot measure objects heavier than 600g you will have to sum up individual pieces and propagate the error. so what would be the mass and the standard error of the cart and the mass
Answers: 3

Physics, 22.06.2019 15:30
What are the similarities & differences between a thermistor and a light dependent resistor in physics?
Answers: 2
You know the right answer?
A 2.5 kg sample of water with an intial temperature of 98o C loses 375,000 J of heat. What is the fi...
Questions

Mathematics, 25.11.2020 08:10


English, 25.11.2020 08:10


Mathematics, 25.11.2020 08:10



Social Studies, 25.11.2020 08:10

Mathematics, 25.11.2020 08:10

Chemistry, 25.11.2020 08:10


Chemistry, 25.11.2020 08:20


Chemistry, 25.11.2020 08:20



Mathematics, 25.11.2020 08:20



Mathematics, 25.11.2020 08:20



