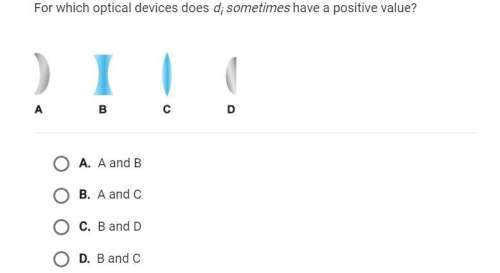
As a bicycle pump inflates a tyre, it pressure rises from 30 kPa to 40 kPa at constant temperature of 30 °C. By assuming the air acts as an ideal gas, calculate the work done per mol of the air.
A. -80.35 J
B. 80.35 J
C. -811.93 J
D. 811.93 J
(please show calculation)

Answers: 2


Another question on Physics

Physics, 22.06.2019 23:00
1which body contains the majority of the mass in the solar system earth jupiter the moon the sun 2 what keeps the objects in the solar system such as planets orbiting around the sun the force of gravity the galaxy exerts on all objects within it including the solar system the great distance between the sun and the planets the sun's nuclear fusion which acts on each object the gravitational force between the sun and each object in the solar system
Answers: 2


Physics, 23.06.2019 06:00
Atoms in a molecule are bonded together by sharing gaining or losing_
Answers: 1

Physics, 23.06.2019 07:30
50 points to who answers this question in a clear and simple explanation : to what hight will a mass of weight of 500 n be raised in 20 seconds by a motor using 4kw of power?
Answers: 1
You know the right answer?
As a bicycle pump inflates a tyre, it pressure rises from 30 kPa to 40 kPa at constant temperature o...
Questions

Mathematics, 05.12.2019 01:31



Geography, 05.12.2019 01:31

Mathematics, 05.12.2019 01:31












English, 05.12.2019 01:31

Business, 05.12.2019 01:31