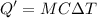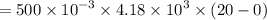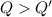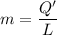Answers: 2


Another question on Physics

Physics, 21.06.2019 13:30
The magnitude of the poynting vector of a planar electromagnetic wave has an average value of 0.939 w/m2 . the wave is incident upon a rectangular area, 1.5 m by 2.0 m, at right angles. how much total electromagnetic energy falls on the area during 1.0 minute
Answers: 1

Physics, 21.06.2019 23:00
Athermometer is removed from a room where the temperature is 70° f and is taken outside, where the air temperature is 10° f. after one-half minute the thermometer reads 60° f. what is the reading of the thermometer at t = 1 min? (round your answer to two decimal places.) ° f how long will it take for the thermometer to reach 30° f? (round your answer to two decimal places.)
Answers: 3

Physics, 21.06.2019 23:00
Acoal-fired plant generates 600 mw of electric power. the plant uses 4.8 x 10^6 kg of coal each day, and the heat of combustion of coal is 3.3x 10^7 j/kg. the steam that drives the turbines is at a temperature of 300° c, and the exhaust water is at 37° c. (a) what is the overall efficiency of the plant for generating electric power? (b) how much thermal energy is exhausted each day? (c) what is the change in entropy of the universe for this heat engine? (d) using the same heat reservoirs, what is the maximum possible (carnot) efficiency for a heat engine?
Answers: 1

Physics, 22.06.2019 02:20
According to newton’s first law of motion, which force is expected to cause a body to accelerate?
Answers: 1
You know the right answer?
A 200 g piece of ice at 0°C is placed in 500 g of water at 20°C. The system is in a container of neg...
Questions

History, 09.07.2019 19:00


English, 09.07.2019 19:00


English, 09.07.2019 19:00

Mathematics, 09.07.2019 19:00




Mathematics, 09.07.2019 19:00



Mathematics, 09.07.2019 19:00


History, 09.07.2019 19:00



Biology, 09.07.2019 19:00

Biology, 09.07.2019 19:00

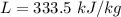

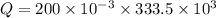
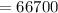 J
J