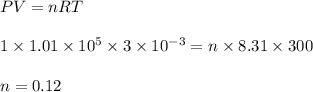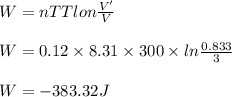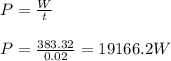Physics, 09.07.2021 22:20 wiredq2049
A cylinder of volume 3 liter has Argon gas initially at 300 K, and 1.00 atm pressure. The piston compresses the gas to a new pressure of 3.60 atm. During this compression, the temperature is maintained constant by using an appropriate heat sink. Find (a) the final volume of the gas, (b) the work done on the gas by the piston, (c) the energy transferred out by heat.(d) If the process takes 20 milliseconds, what is the power

Answers: 2


Another question on Physics


Physics, 22.06.2019 11:00
A0.580-kg rock is tied to the end of a string and is swung in a circle with a radius of 0.500 meters. the velocity of the rock is 4.50 m/s. what is the centripetal force acting on the rock? 15.5 n 5.22 n 69.8 n 23.5 n
Answers: 1

Physics, 22.06.2019 14:10
The number of passengers who arrive at the platform of a subway station for the 10 am train is a random variable with a mean of 120 and a variance of 16. find the lower bound of the probability that there will be between 100 and 140 passengers (round off to second decimal place).
Answers: 3

Physics, 22.06.2019 16:00
The solid that is formed and usually sinks to the bottom of a solution is the
Answers: 2
You know the right answer?
A cylinder of volume 3 liter has Argon gas initially at 300 K, and 1.00 atm pressure. The piston com...
Questions



Biology, 15.09.2019 17:10





Mathematics, 15.09.2019 17:10

Physics, 15.09.2019 17:10

Mathematics, 15.09.2019 17:10

Mathematics, 15.09.2019 17:10

Mathematics, 15.09.2019 17:10

History, 15.09.2019 17:10

Mathematics, 15.09.2019 17:10





Mathematics, 15.09.2019 17:10