
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300 K. The temperature of the gas in the first cylinder is increased to 412 K at constant volume by doing work W1 and transferring energy Q1 by heat. The temperature of the gas in the second cylinder is increased to 412 K at constant pressure by doing work W2 while transferring energy Q2 by heat.
Required:
Find ÎEint, 1, Q1, and W1 for the process at constant volume.

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:30
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies.
Answers: 3

Physics, 22.06.2019 04:30
In a voltaic cell, electrons flow from the (positive/negative) to the (positive/negative) terminal.
Answers: 1

Physics, 22.06.2019 12:30
Write a full page that sumerizes thermodynamics it’s from the website visionlearnig
Answers: 1

Physics, 22.06.2019 18:30
Suppose you plot the distance traveled by an object at various times and you discover that the graph is not a straight line. what does this indicate about the object's acceleration?
Answers: 3
You know the right answer?
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300...
Questions


Mathematics, 12.01.2021 02:50

Mathematics, 12.01.2021 02:50

History, 12.01.2021 02:50

Physics, 12.01.2021 02:50

Mathematics, 12.01.2021 02:50



Mathematics, 12.01.2021 02:50

Mathematics, 12.01.2021 02:50

Mathematics, 12.01.2021 02:50

History, 12.01.2021 02:50

Mathematics, 12.01.2021 02:50



Mathematics, 12.01.2021 02:50

Biology, 12.01.2021 02:50

Mathematics, 12.01.2021 02:50


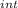 ,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J
,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J  = 300 K, T
= 300 K, T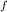 = 412 K, n = 0.7 mol
= 412 K, n = 0.7 mol 

