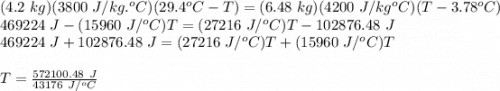An ice chest at a beach party contains 12 cans of soda at 3.78 °C. Each can of soda has a mass of 0.35 kg and a specific heat capacity of 3800 J/(kg C°). Someone adds a 6.48-kg watermelon at 29.4 °C to the chest. The specific heat capacity of watermelon is nearly the same as that of water. Ignore the specific heat capacity of the chest and determine the final temperature T of the soda and watermelon in degrees Celsius.

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:30
From 0 to 5 seconds john pushed a box 5 meters. from 5 to 10 seconds, paul pushed the same box another 5 meters. who did more work? a. john b. paul c. john and paul did the same amount of work.
Answers: 1

Physics, 22.06.2019 07:50
The ratio of lift to drag l/d for a wing or airfoil is an important aerodynamic parameter, indeed, it is a direct measure of the aerodynamic efficiency of the wing. if a wing is pitched through a range of angle of attack, l/d first increases, then goes through a maximum, and then decreases. consider an infinite wing with an naca 2412 airfoil. estimate the maximum value of l/d. assume that the reynolds number is 9x10^6.
Answers: 2

Physics, 22.06.2019 09:00
The pressure proportional to the area a- inversely b- directly c- increase d-decrease
Answers: 2

Physics, 22.06.2019 10:00
Abookcase has a mass of 38 kg and the coefficient of friction between it and the floor is 0.82 what is the maximum force of friction between the bookcase and the floor a 372n b 305n c 412n d 449n
Answers: 1
You know the right answer?
An ice chest at a beach party contains 12 cans of soda at 3.78 °C. Each can of soda has a mass of 0....
Questions




Mathematics, 07.12.2021 01:00



English, 07.12.2021 01:00

Mathematics, 07.12.2021 01:00


English, 07.12.2021 01:00


Arts, 07.12.2021 01:00



Spanish, 07.12.2021 01:00

Social Studies, 07.12.2021 01:00



Mathematics, 07.12.2021 01:00

English, 07.12.2021 01:00

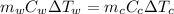
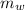 = mass of watermelon = 6.48 kg
= mass of watermelon = 6.48 kg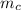 = mass of cans = (12)(0.35 kg) = 4.2 kg
= mass of cans = (12)(0.35 kg) = 4.2 kg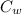 = specific heat capacity of watermelon = 3800 J/kg.°C
= specific heat capacity of watermelon = 3800 J/kg.°C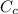 = specific heat capacity of cans = 4200 J/kg.°C
= specific heat capacity of cans = 4200 J/kg.°C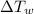 = Change in Temprature of watermelon = 29.4°C - T
= Change in Temprature of watermelon = 29.4°C - T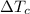 = Change in Temperature of cans = T - 3.78°C
= Change in Temperature of cans = T - 3.78°C