
Physics, 01.07.2021 15:30 kordejah348
A 3.10 mol sample of an ideal diatomic gas expands adiabatically from a volume of 0.1550 m3 to 0.742 m3 . Initially the pressure was 1.00 atm.(a) Determine the initial and final temperatures. initial Kfinal K(b) Determine the change in internal energy. J(c) Determine the heat lost by the gas. J(d) Determine the work done on the gas. J

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:30
Heena wants to measure the volume of a ball that is 24 cm across. how should she set up her equation?
Answers: 1

Physics, 22.06.2019 05:00
Which statements describe the movement of ocean currents around the globe? check all that apply. strong winds force warm water to sink to the ocean floor. the coriolis effect causes warm and cold water to mix. cool dense water sinks to the ocean floor. warm water replaces cool surface water. wind blowing parallel to the shore causes upwelling of cool water.
Answers: 1


Physics, 22.06.2019 12:30
Matter is needed to transfer thermal energy bya. conductionb. convectionc. radiation d. both a & b.
Answers: 1
You know the right answer?
A 3.10 mol sample of an ideal diatomic gas expands adiabatically from a volume of 0.1550 m3 to 0.742...
Questions

English, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31







History, 21.11.2019 01:31



Biology, 21.11.2019 01:31

Biology, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31






 = 1 atm = 101325 pascal
= 1 atm = 101325 pascal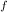 = ?
= ?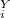 = P
= P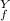
 V
V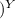
 = nC
= nC ΔT
ΔT

