
Physics, 08.06.2021 01:00 gmoney1973
The liquid and gaseous state of hydrogen are in thermal equilibrium at 20.3 K. Even though it is on the point of condensation, model the gas as ideal and determine the most probable speed of the molecules (in m/s). What If? At what temperature (in K) would an atom of xenon in a canister of xenon gas have the same most probable speed as the hydrogen in thermal equilibrium at 20.3 K?

Answers: 1


Another question on Physics


Physics, 22.06.2019 08:00
Ms.hidalgo opens the door to her classroom. her classroom is 60 degrees and the air outside is 80. predict what will happen using your knowledge of how heat flows.
Answers: 2

Physics, 22.06.2019 19:00
Achild has maximum walking speed of 1.6 m/s. what is the length of the child's legs? 0.26 m
Answers: 2

Physics, 22.06.2019 22:50
When a pair of 10-n forces act on a box of candy, the net force on the box is a 20 n. b about 14 n. c zero. d any of the above depending on the directions of forces.
Answers: 1
You know the right answer?
The liquid and gaseous state of hydrogen are in thermal equilibrium at 20.3 K. Even though it is on...
Questions

Physics, 27.07.2019 10:20

History, 27.07.2019 10:20


Social Studies, 27.07.2019 10:20


Chemistry, 27.07.2019 10:20


Mathematics, 27.07.2019 10:20

Health, 27.07.2019 10:20

Mathematics, 27.07.2019 10:20

Social Studies, 27.07.2019 10:20


Arts, 27.07.2019 10:20


Computers and Technology, 27.07.2019 10:20

Geography, 27.07.2019 10:20

English, 27.07.2019 10:20



Biology, 27.07.2019 10:20

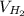 = √( 2RT /
= √( 2RT / 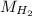 )
)

