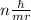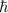Physics, 12.05.2021 01:00 payshencec21
The key insight that Bohr introduced to his model of the atom was that the angular momentum of the electron orbiting the nucleus was quantized. He introduced the postulate that the angular momentum could only come in quantities of nh/(2π), where h is Planck's constant and n is a nonnegative integer (0,1,2,3,…). Given this postulate, what are the allowable values for the velocity v of the electron in the Bohr atom? Recall that, in circular motion, angular momentum is given by the formula L= mvr.

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:30
Which of the following statements is true about women and minorities in early psychology? a. in the early 20th century, numerous graduate schools recruited women and minorities to study psychology. b. opportunities in higher education were limited for women and minorities in the early 20th century due to discrimination. c. despite some obstacles, there were numerous employment opportunities for women and minorities. d. women and minorities were often selected to do academic research in an effort to make the field more diverse.
Answers: 3

Physics, 22.06.2019 11:20
Wave functions describe orbitals in a hydrogen atom. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 2: the quantum number l can have values from to . the total number of orbitals possible at the n = 2 energy level is .
Answers: 3


Physics, 22.06.2019 18:00
Which statement is the best definition of an atom? a) anything that has mass and occupies space. b) the smallest particle that has the properties of an element. c) a substance that cannot be broken down chemically into simpler substances. eliminate d) the smallest unit of a substance that exhibits all of the properties characteristic of that substance.
Answers: 2
You know the right answer?
The key insight that Bohr introduced to his model of the atom was that the angular momentum of the e...
Questions




Physics, 25.01.2022 02:50

Mathematics, 25.01.2022 02:50




Physics, 25.01.2022 02:50

Mathematics, 25.01.2022 02:50

Biology, 25.01.2022 02:50

Mathematics, 25.01.2022 02:50

Biology, 25.01.2022 02:50

Mathematics, 25.01.2022 02:50



Physics, 25.01.2022 02:50


Business, 25.01.2022 02:50

Mathematics, 25.01.2022 02:50