Ineed !
a 50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a co...

Physics, 14.12.2019 02:31 claytonashley30
Ineed !
a 50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorimeter filled with water. the calorimeter and the water have a combined mass of 250.0 g and an overall specific heat of 1.035 cal/g•°c. the initial temperature of the calorimeter is 10.00°c. the system reaches a final temperature of 11.08°c when the metal is added. what is the specific heat of the

Answers: 2


Another question on Physics


Physics, 22.06.2019 11:30
While you are driving in the lane next to the curb on a multi-lane road the car on your left suddenly moves toward you lane. they are about toy crash into your front fender. you
Answers: 2

Physics, 22.06.2019 14:00
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 22.06.2019 14:50
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2
You know the right answer?
Questions


Mathematics, 17.12.2020 20:10




Biology, 17.12.2020 20:10


English, 17.12.2020 20:10

Geography, 17.12.2020 20:10

History, 17.12.2020 20:10

SAT, 17.12.2020 20:10







English, 17.12.2020 20:10

Social Studies, 17.12.2020 20:10

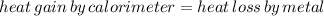
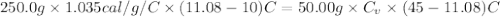
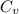 is the specific heat of the unknown metal. Solving this gives us the specific heat of the metal:
is the specific heat of the unknown metal. Solving this gives us the specific heat of the metal: