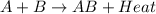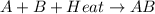Physics, 26.12.2019 08:31 akeemsimpson15p2034o
In a given chemical reaction, the energy of the products is less than the energy of the reactants. which statement is true for this chemical reaction?
a. energy is absorbed in the reaction.
b. energy is released in the reaction.
c. there is no transfer of energy in the reaction.
d. energy is lost in the reaction.

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:50
Identify the arrows that show the correct direction of heat transfer. 66°f 112°f 98°f
Answers: 2

Physics, 21.06.2019 20:00
The sun generates both mechanical and electromagnetic waves. which statement about those wave is true?
Answers: 1

Physics, 21.06.2019 22:30
An engineer designs a roller coaster so that a car travels horizontally for 162 ft, then climbs 127 ft at an angle of 31.0° above the horizontal. it then moves 127 ft at an angle of 46.0° below the horizontal. if we take the initial horizontal motion of the car to be along the +x-axis, what is the car's displacement? (give the magnitude of your answer, in ft, to at least four significant figures and give the direction of your answer in degrees counterclockwise from the +x-axis.)
Answers: 1

Physics, 22.06.2019 03:00
Isla’s change in velocity is 30 m/s, and hazel has the same change in velocity. which best explains why they would have different accelerations? isla had negative acceleration, and hazel had positive. isla had a different time than hazel. isla had positive acceleration, and hazel had negative. isla went a farther distance than hazel.
Answers: 1
You know the right answer?
In a given chemical reaction, the energy of the products is less than the energy of the reactants. w...
Questions


Mathematics, 26.08.2019 17:30


Mathematics, 26.08.2019 17:30


Biology, 26.08.2019 17:30



Computers and Technology, 26.08.2019 17:30