Physics, 19.09.2019 19:30 tinytoonjr9510
1. an ideal gas expands at a constant pressure of 6.00 105 pa from a volume of 1.00 m3 to a volume of 4.00 m3 and then is compressed to one-third that pressure and a volume of 2.50 m3 as shown in the figure below before returning to its initial state. how much work is done in taking a gas through one cycle of the process shown in the figure?
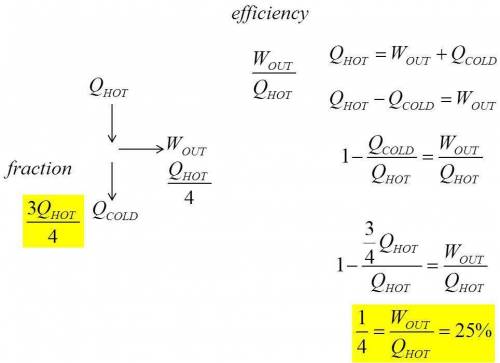
2. the work done by an engine equals one-fourth the energy it absorbs from a reservoir.
(a) what is its thermal efficiency?
(b) what fraction of the energy absorbed is expelled to the cold reservoir?

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:50
Ammonia enters the expansion valve of a refrigeration system at a pressure of 10 bar and a temperature of 18oc and exits at 6.0 bar. the refrigerant undergoes a throttling process. determine the temperature, in oc, and the quality of the refrigerant at the exit of the expansion valve.
Answers: 3

Physics, 22.06.2019 04:30
In a voltaic cell, electrons flow from the (positive/negative) to the (positive/negative) terminal.
Answers: 1

Physics, 22.06.2019 04:30
In a system, when potential energy decreases, then entropy also decreases. true false
Answers: 3

Physics, 22.06.2019 16:00
In which of the following is positive work done by a person on a suitcase
Answers: 1
You know the right answer?
1. an ideal gas expands at a constant pressure of 6.00 105 pa from a volume of 1.00 m3 to a volume o...
Questions




English, 29.01.2020 08:02

Mathematics, 29.01.2020 08:02

Health, 29.01.2020 08:02


Chemistry, 29.01.2020 08:02

Mathematics, 29.01.2020 08:02

Social Studies, 29.01.2020 08:02



Physics, 29.01.2020 08:02


Mathematics, 29.01.2020 08:02

Mathematics, 29.01.2020 08:02

Physics, 29.01.2020 08:02


Mathematics, 29.01.2020 08:02